🚨MEGA BOMBSHELL: BIOMARKER SCAMS🚨 Critique of Gene Editing and Biomarker Hype
PubMed has catalogued 1.2 million biomarker studies. Yet fewer than 1 percent of breast‑cancer biomarkers ever make it into clinical care.
When ‘precision editing’ means stabbing bread and hoping for toast.
Yes, wishful slicing — and other dumb things people do for money and status.
In 2019, scientists touted CRISPR‑edited hornless dairy calves as clean, precise genetic engineering. But an FDA review later found antibiotic‑resistance genes from bacterial plasmids hidden in the calves—derailing the entire narrative of 'seamless gene editing.'
If Theranos taught us anything, it’s that measuring more doesn’t mean we know more.
And yet we’re now applying the same logic to DNA, calling it “editing,” and selling it as salvation.
“Theranos Founder Elizabeth Holmes Charged With ‘Massive Fraud.’” – Time, Mar 2018: SEC details on the fraud charges and Holmes’s settlement.
Genes are just another biomarker — messy, context-dependent, and wildly overhyped. Like cholesterol or serotonin, they offer the illusion of precision in a sea of biological chaos.
🚨"Biomarkers aren’t niche—they’re everywhere:
Over half of all oncology trials now depend on them, and PubMed has catalogued 1.2 million biomarker studies. Yet fewer than 1 percent of breast‑cancer biomarkers ever make it into clinical care. A clear sign of how deeply they're embedded in research informatics.bmj.com. Yet despite the volume, translation to real-world use remains limited: only about 0.9% of breast cancer recurrence biomarkers identified in research were recommended for clinical use bmcmedicine.biomedcentral.com.
In oncology, around 55% of clinical trials in 2018 included at least one biomarker, up from just ~15% in 2000 — a huge rise driven by the push for precision medicine bmcmedresmethodol.biomedcentral.com+2lek.com+2linkedin.com+2.
That works out to a compound annual growth rate of ~17% for biomarker‑linked trials over nearly two decades lek.com.
It's a hot research trend, often tied to funding, pharma, or tech platforms.
Most biomarkers fail due to:
Poor reproducibility
Overfitting in small datasets
Context-dependence (i.e. working in one population but not others)
Weak causal evidence
Biomarker studies are a big part of the reproducibility crisis—most rely on small, single‑cohort designs that often fail when retested in larger, more diverse populations.
So: we study a lot, publish a lot, and market a lot — but almost nothing gets translated into trustworthy clinical tools.
Let’s start here: hCG biomarkers.
Biomarkers Aren’t Levers: The hCG Fallacy Behind Anti-Fertility Vaccines
In the world of biotech and population control experiments, the idea that you can switch off fertility by targeting human chorionic gonadotropin (hCG) has gained traction. It’s the hormone that shows up early in pregnancy — so, they assume, if you vaccinate women against hCG, you can prevent pregnancy. Neat, right?
Except the entire premise is wrong.
It’s another case of mistaking a signal for a cause — a classic biomarker fallacy.
The False Logic Behind Anti-hCG Vaccines
The argument goes:
hCG appears after fertilisation → pregnancy proceeds
So, block hCG = block pregnancy
Therefore, hCG must cause pregnancy
But what if that’s not true?
What if hCG is just the body’s way of marking time — a biological timestamp, not a command switch?
What hCG Actually Does
hCG is produced by the embryo shortly after fertilisation. Scientists think it tells the corpus luteum to keep producing progesterone, which stabilises the uterine lining and supports implantation.
Scientists think removing hCG can disrupt early pregnancy. But that doesn’t mean hCG causes pregnancy. It maybe just coordinates the next steps, like a thermostat or a green light. You can cause a crash by smashing the traffic light — but that doesn’t make the light the engine.
This distinction matters. Because using hCG as a vaccine target is a sledgehammer solution built on a reductionist fantasy.
Wait — What About β-hCG Specifically?
Good question. Everything above applies directly to β-hCG, which is the specific subunit targeted in pregnancy tests, anti-fertility vaccines, and tumor diagnostics. Unlike the alpha subunit (shared with other hormones like LH and TSH), β-hCG is considered "pregnancy-specific" — and that’s exactly why it was chosen as a vaccine target. But "specific" doesn’t mean "causal." The body uses β-hCG as a timestamp, not a master switch. It can appear in viable pregnancies, failed pregnancies, men, and cancer patients alike. So yes — this isn’t just about hCG in general. It’s the misuse of β-hCG in particular that reveals the hollowness of the whole biomarker-as-lever fantasy.
The Evidence: Why hCG Is Not a Fertility Switch
Let’s look at the real-world data.
1. Huge variability in hCG — even in healthy pregnancies
Studies show up to 20-fold variation in early hCG levels between women — even when pregnancies are completely viable.
Source:
Cole LA et al., Clinical Chemistry, 2005
https://academic.oup.com/clinchem/article/51/9/1570/5620402
Translation:
There is no magic “fertility threshold” for hCG.
Low hCG doesn’t mean no pregnancy — it just means every body runs on a slightly different clock.
2. High hCG levels in failed pregnancies
Ectopic and molar pregnancies can produce very high hCG levels — but the embryo is non-viable or absent.
Source:
Barnhart KT, NEJM, 2009
https://www.nejm.org/doi/full/10.1056/nejmra0804661
Translation:
High hCG doesn’t mean a healthy baby.
It’s just a sign of trophoblastic activity, not successful reproduction.
3. Fertility persists despite anti-hCG antibodies
Some women in vaccine trials developed antibodies to hCG — but still conceived.
Source:
Talwar GP et al., Immunological Reviews, 1999
https://pubmed.ncbi.nlm.nih.gov/10319258/
Translation:
The body has redundant systems.
Disrupting hCG may reduce fertility temporarily, but it’s not a sterilisation switch.
4. hCG exists outside of pregnancy
Low levels of hCG can be found in men and non-pregnant women, produced by the pituitary and other tissues.
Source:
Cole LA, Reproductive Biology and Endocrinology, 2009
https://rbej.biomedcentral.com/articles/10.1186/1477-7827-7-22
Translation:
hCG isn’t even pregnancy-specific.
It’s a contextual marker, not a magical fertility hormone.
Analogy: Fever ≠ Infection
Fever appears during infection — but lowering the fever doesn’t kill the pathogen.
Same with hCG:
Blocking it doesn’t eliminate fertility — it just scrambles the body’s timing.
You didn’t prevent pregnancy.
You just broke the clock.
Final Thoughts: What “Works” Isn’t Always Smart
MAYBE, anti-hCG vaccines can somehow interfere with pregnancy — but not because hCG is a master key. They work the same way tearing out the fire alarm works to “prevent fire detection.”
This isn’t precision medicine.
It’s a blunt-force hack that hijacks a timing cue and MAYBE sabotages the downstream process.
And when it’s done without full understanding — or without informed consent — it’s not science.
It’s bio-political control.
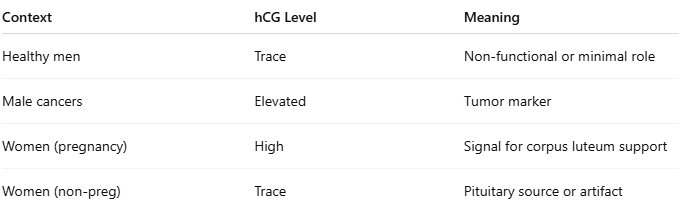
Do men produce hCG or β-hCG?
Yes — in trace amounts.
The pituitary gland can secrete very low levels of hCG, especially in older men or those with certain hormonal shifts.
In healthy men, baseline levels are usually below 5 mIU/mL — often undetectable without ultrasensitive tests.
hCG can also appear in testicular tissue, and is sometimes elevated in certain cancers.
When is hCG found in men?
1. Normal physiology
Pituitary-derived hCG is part of the body's hormonal orchestra.
It may play a minor regulatory role in gonadal function, but it's not essential for fertility.
2. Pathological conditions (red flag)
Elevated β-hCG is a tumor marker in men:
Testicular cancer (especially choriocarcinoma)
Germ cell tumors
Some gastrointestinal or lung cancers
In these cases, doctors use β-hCG as a diagnostic and tracking tool, not as a fertility indicator.
Even though β‑hCG spikes in some cancers, this biomarker also pops up in older men, people who smoke pot, and nearly a dozen non‑trophoblastic tumors—making it a correlated byproduct, not a cause‑targeted tool.
Gloucestershire NHS (pathology notes): "As with most tumour markers there is a high incidence of false positive and false negative results; hCG is most appropriately used in monitoring … once diagnosis … has been made."
Clinical Chemistry (2023): β‑hCG is used off‑label in tumor settings—no FDA-approved oncology hCG test exists.
American Journal of Clinical Pathology (2018 case): "beta‑hCG can be rarely expressed by non‑trophoblastic tumors of the bladder, breast, lung, gastrointestinal tract, etc."
Clinical Chemistry (2023 review): "Ectopic production of several hCG variants has been well documented in … non‑trophoblastic (e.g., breast, ovarian, and colorectal) disease … no hCG test method is FDA‑approved for oncology…it is considered ‘off‑label’"
Caring for the Female Cancer Patient (Cambridge): "Other non‑pregnant or false‑positive causes of serum β‑hCG testing include pituitary hormone production in perimenopausal or postmenopausal women…"
CancerPoints.com: "Beta HCG. Marijuana use can increase beta HCG levels… Older men are more likely to have false‑positive elevated β‑hCG."
So what does this mean?
hCG is not exclusive to pregnancy or women — it’s part of the broader glycoprotein hormone family.
Its presence in men shows that biomarkers like hCG exist across contexts, but with different meanings.
That reinforces my core point:
Just because a molecule appears in one context (like pregnancy) doesn’t mean it causes that event.
AGAIN —> hCG:
Varies wildly between individuals
Is present in men, non-pregnant women, even in cancer
Is not required in strict amounts for pregnancy to proceed
Can be sky-high in failed pregnancies
Can be low or undetectable in viable ones
Has non-reproductive roles and isn't exclusive to gestation
🚨Reevaluating Immune Biomarkers // The Case for Disease Tolerance in Clinical Assessment
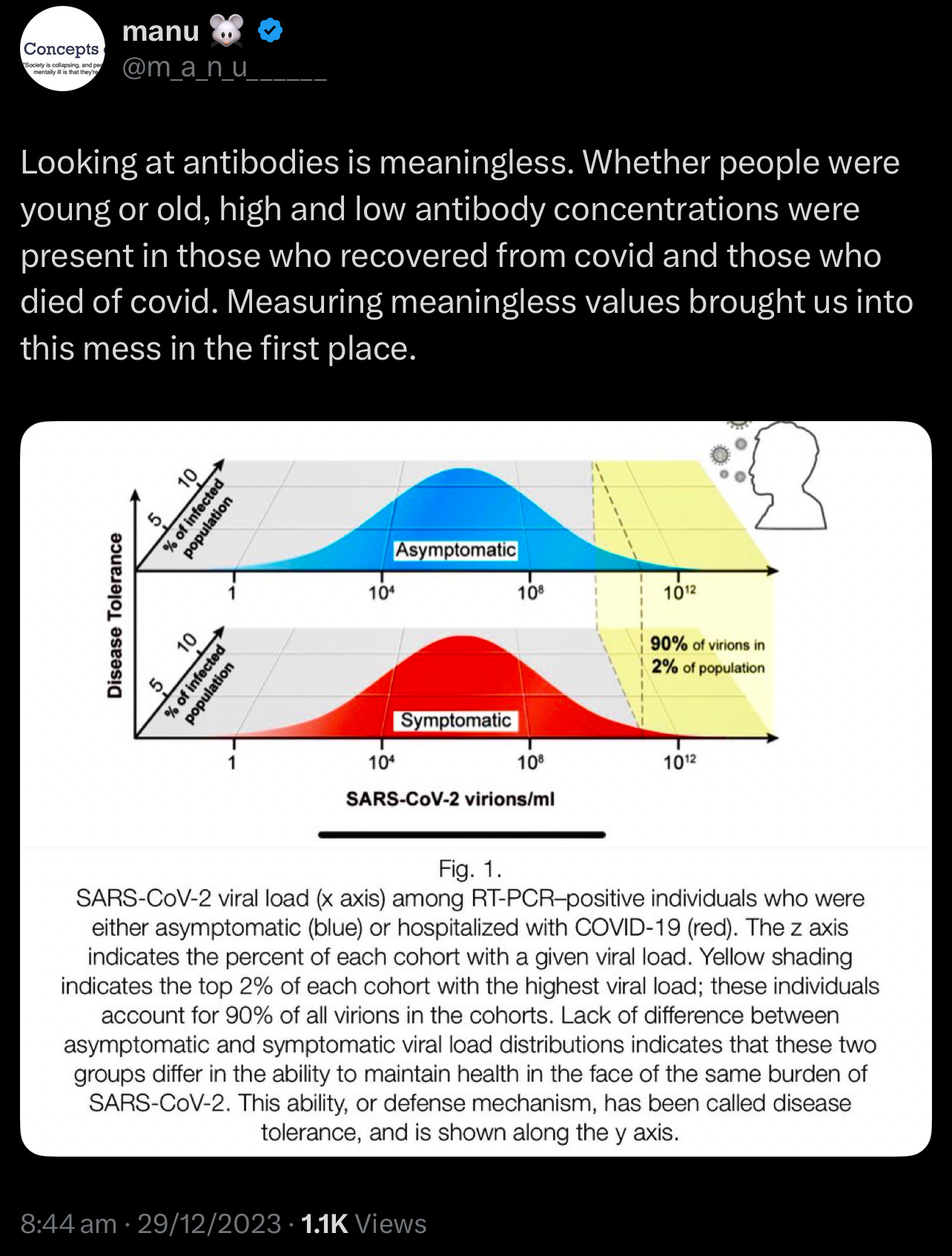
Traditional immunological assessments have long relied on measuring antibody and T cell levels. However, emerging evidence suggests that these metrics alone may not consistently predict clinical outcomes. Studies have shown that antibody and T cell counts can vary significantly among individuals, often lacking a direct correlation with disease severity or patient prognosis. This observation has led to a growing interest in the concept of disease tolerance—the body's ability to minimize tissue damage and maintain internal stability during infections. Unlike traditional immune responses that focus on eliminating pathogens, disease tolerance emphasises mechanisms that control damage, explaining why some individuals experience mild symptoms despite similar pathogen exposures. This paradigm shift offers a more comprehensive understanding of immune competence and resilience.
This challenges the established understanding of the role of antibodies and T cells in immune responses!
Lymphocytes are a type of white blood cell essential to the immune system, and T cells are a subset of lymphocytes. my.clevelandclinic.org
The study by Chen et al. found that lymphocyte counts below 2.99 ×10^9/L on day 7 after sepsis onset were associated with an 85.42% sensitivity for predicting 28-day mortality. However, interpreting lymphocyte counts can be challenging due to the broad physiological ranges observed in healthy individuals. For example, CD4+ T cell counts typically vary from 500–1500 cells/μL, making it difficult to establish definitive thresholds for "normal" or "abnormal" levels. my.clevelandclinic.org
Additionally, factors such as immunosenescence (the gradual decline of immune function with age), stress hormones, and genetic polymorphisms can further complicate the relationship between T cell metrics and health outcomes. my.clevelandclinic.org
Sources:
T cell-mediated Immune response and correlates of inflammation and clinical outcome in COVID-19
Disease tolerance: a protective mechanism of lung infections
Disease tolerance and immunity in host protection against infection
Limitations of Current Immune Biomarkers
Context-Dependent Variability
The same T cell count may confer protection or pathology depending on microenvironmental cues. During malaria reinfection, CD4+ T cells transition from pro-inflammatory cytokine producers to regulators of tissue repair, illustrating that functional status—not merely quantity—determines outcome 2. Similarly, sepsis survivors showed monocyte repopulation by day 7 (AUC=0.834 for mortality prediction), but baseline counts lacked prognostic value 3. This temporal and contextual variability undermines static measurements.
Disconnect Between Pathogen Load and Morbidity
Chronic infections like malaria and tuberculosis persist despite robust antibody and
T cell responses. In mouse malaria models, survival improved not through enhanced parasite clearance, but via myeloid reprogramming that reduced endothelial activation and iron dysregulation 2. These tolerance mechanisms operated independently of pathogen load, highlighting a critical blind spot in traditional biomarkers.
Disease Tolerance as an Alternative Framework
Defining Tolerance in Host Defense
Disease tolerance minimises fitness costs of infection through:
Tissue Resilience: Enhancing cellular stress resistance (e.g., antioxidant upregulation)
Damage Control: Suppressing immunopathology (e.g., regulatory T cell induction)
Metabolic Adaptation: Reprogramming energetics to sustain vital functions 2 4
Unlike resistance, tolerance does not directly reduce pathogen burden, making it invisible to conventional immune assays. However, its clinical relevance is profound: during Plasmodium reinfection, mice lacking tolerance mechanisms succumbed to cerebral malaria despite equivalent parasitemia to tolerant counterparts 2.
Enhance tissue resilience through dietary modifications and reduce exposure to toxins to bolster cellular stress resistance. Adopting an anti-inflammatory diet can modulate inflammatory processes and improve health outcomes!
Why Genes Became Biomarkers
Genetic sequences and mutations got labeled as biomarkers because they correlate with disease risk. BRCA1, APOE4, TP53 — they don’t cause disease directly, but they show up often enough in the sick that researchers started using them like warning lights. It was tempting: DNA looked objective, measurable, and predictive. But correlation ≠ causation… and biomarkers aren’t blueprints — they’re more like vague street signs.
Hype vs. Truth in Metaphors: CRISPR is not like editing a Word document; it’s more like triggering a mutation and hoping for the best. Calling CRISPR ‘editing’ is misleading – it’s less a precise copy-editor and more a molecular shotgun, peppering the genome and relying on chance to hit the target just right news-medical.net.
Cautionary Quote: As one geneticist put it, there’s no evidence gene editing causes fewer unintended mutations than old-fashioned breeding – it may in fact cause more in some cases greens-efa.eu. The supposed sniper rifle of genetics can act like a blunderbuss.
Hype Cycle Narrative: CRISPR has climbed the peak of inflated expectations – Nobel prizes, magazine covers, ‘designer baby’ fears and dreams – and is now descending into the trough of disillusionment. The question is whether it will emerge on the slope of enlightenment with sustainable, proven applications, or be remembered as another over-hyped biotech fad.
Historical Analogy: Back then, the Human Genome Project’s completion led to bold claims that we’d soon have personalized medicine for all. Similarly, CRISPR’s discovery led to bold claims of precise genetic cures. In both cases, the “last mile” – turning a genomic insight or edit into a safe, effective therapy – turned out to be the hardest part. If the 2000s taught us that having a genome sequence didn’t automatically deliver cures, the 2020s are teaching us that being able to tweak a genome isn’t the same as knowing how to improve an organism.
The Oversell Problem: The biggest danger for gene editing may be its own hype. When expectations are set unrealistically high – miracle cures right around the corner – the public and investors can become jaded or angry when reality intervenes. We saw this with biomarkers, with stem cells, and now we see it with CRISPR: the technology is real, but so are its limits, and overselling it can do lasting harm.
Critiques of Modern Gene Editing:
Hype vs. Reality
🚨Precision? Even the “molecular scalpel” cuts the wrong DNA half the time.
🚨CRISPR made hundreds of unintended edits in supposedly “precise” rice plants. Precision, meet chaos.
☝️That’s a scalpel poking a beehive☝️
By placing a scalpel at a beehive, the image sets up a visual metaphor for how an exacting genetic tool is being applied to a volatile living system. The scalpel’s sharp focus and gleam convey human intent to carefully “fix” nature, while its incongruous presence in a beehive signals the underestimation of nature’s complexity – a quiet before an impending storm of bees.
☝️Bees swarming out of their hive in a chaotic flurry – representing the unleashed consequences of poking the “genetic beehive.” The image shows the moment the scalpel tip pricks the hive, angry bees erupt in all directions, much like unpredicted mutations or cascading effects when a genome is edited. This swarm visualises how biological systems can respond in uncontrolled ways: each bee is a metaphor for a rogue outcome, an “off-target” edit or a ripple effect beyond the scientist’s intent. The chaos of thousands of bees overwhelming the scene highlights the core message that gene editing is not a neat, isolated intervention – it’s more like startling an entire colony. The contrast between the bees’ erratic movement and the scalpel’s precision underscores a satirical point: tinkering with DNA can be like performing surgery on a beehive and expecting total cooperation from the bees.
1. “Gene Editing” vs. Natural Mutations and Imprecise Methods
Many so-called “gene editing” breakthroughs are not precise DNA edits in the way people imagine (like editing text). Instead, they often involve either selecting pre-existing genetic variants or making random changes and then picking the desired outcome. In agriculture and animal breeding, for example, gene editing is frequently used to replicate natural mutations that could have been obtained through traditional breeding. A notable case is the creation of hornless cattle: Researchers used gene editing (TALENs/CRISPR) to introduce a mutation already found in some cattle breeds (the polled mutation in Angus cows) into dairy cattle. A company executive even claimed the gene-edited animal was essentially the same as one bred conventionally thecounter.org. This underscores that the edit was not a novel invention, but a sped-up form of selective breeding. (Ironically, regulators later found these “precisely edited” calves carried unexpected antibiotic-resistance genes from the bacterial editing plasmid, revealing imprecision and contamination thecounter.orgthecounter.org.)
Modern gene editing tools like CRISPR-Cas9 are often described as molecular scissors or even “find-and-replace” tools. In reality, CRISPR is a cut-and-damage mechanism that relies on the cell’s error-prone repair process to achieve a change edepot.wur.nl. The intended DNA cut can be targeted to a specific gene, but what happens after the cut is probabilistic. As one review put it, “though it is called ‘editing’, CRISPR… [has] no DNA repair function… When repairs are made to the DNA at the cut site… they are largely out of the control of the experimenter. Ten independent editing events will therefore give ten different mutations at the same location in the genome.” news-medical.net In other words, researchers often create many mutations and then screen for the desired one – a process not so different from older mutagenesis breeding, just with more guidance as to where the cut happens. Indeed, a molecular geneticist noted there is no evidence that gene editing causes fewer off-target mutations than conventional breeding or radiation/chemical mutagenesis techniques greens-efa.eu. On the contrary, CRISPR edits can generate large deletions, insertions, and rearrangements in DNA that would never occur naturally, belying the popular notion of CRISPR’s precision greens-efa.eu.
Examples in Animals: Chinese scientists famously used CRISPR to create double-muscled beagle dogs by knocking out the myostatin gene – essentially mimicking the naturally occurring mutation that gives bully whippet dogs and Belgian Blue cattle their muscular physique sciencenews.org. The outcome was only partly successful: out of 27 embryos implanted, only two puppies were born with the edited gene (one had the muscle mutation in every cell, another was a mosaic) sciencenews.org. The rest had no edit or partial edits, highlighting low efficiency. As the researchers admitted, the “low number of puppies born with edited genes” shows that the CRISPR process “is not very efficient” in practice sciencenews.org. Such cases show that what’s often labeled as a gene-editing triumph (a buff dog, a hornless cow, a disease-resistant plant) may be less a surgical rewrite of a gene and more a lottery of mutations and selections – “precision” only in retrospect once a desirable mutant is identified.
2. Limited Understanding of Genes and Unintended Consequences
A core problem is that scientists still do not fully understand the function of most genes or the complex genetic networks in cells. Many gene-trait associations are discovered through genome-wide association studies (GWAS) or other correlational methods, which identify genetic variants linked to diseases or traits. But association is not causation edepot.wur.nl. For complex traits (height, diabetes, behavior, etc.), dozens or hundreds of gene variants may be involved, and their effects depend on context and environment. Editing one “candidate” gene often fails to produce the expected outcome because the genetic architecture is so intricate. As one analysis noted, identifying which DNA variants actually cause a given trait “has been difficult,” and moreover, most variants linked by studies lie in noncoding (regulatory) regions, so their functional impact is hard to determine edepot.wur.nl. In short, we often don’t know what we’re truly tweaking. The very concept of “a gene for X” is much blurrier than early genetics suggested – modern genomics shows genes interact in networks, encode multiple products, and can have multiple roles edepot.wur.nledepot.wur.nl. The simplistic idea of a gene as a single, discrete instruction is outdated, yet much of the CRISPR hype glosses over this complexity edepot.wur.nledepot.wur.nl.
Because of this limited understanding, attempts to edit genes can lead to surprises. A stark example is the case of the CRISPR baby experiment in 2018. A researcher edited the CCR5 gene in human embryos, aiming to replicate a known mutation (CCR5-Δ32) that confers HIV resistance. Not only was this experiment widely condemned as unethical, it also likely failed on a technical level: the babies ended up with new, rare mutations in CCR5, not the exact Δ32 variant journals.plos.org. The scientist did not even attempt to precisely insert the known protective mutation via HDR (homology-directed repair); instead he relied on error-prone editing to knock out the gene, yielding irregular indels journals.plos.org. Experts pointed out that other CCR5-null mutations cannot be assumed to mimic the effects of the well-studied Δ32 allele journals.plos.org. In fact, knocking out CCR5 in humans could have unintended consequences: CCR5 has normal immune functions (it’s a receptor on white blood cells), and CCR5-knockout mice show altered immune responses and higher susceptibility journals.plos.org. Thus, the attempt to create “HIV-proof” children was not only premature ethically, but scientifically naive – it illustrated how poorly we predict a gene’s ripple effects on physiology. The outcome (two baby girls with unknown mutations and mosaic editing) fell far short of the promised precision.
Unintended effects aren’t limited to human cases; they are common across gene editing experiments. Even “on-target” edits – changes at the intended gene – can have unpredicted outcomes. For instance, when researchers edited pigs extensively to create organs safe for transplantation, they deleted dozens of viral genes and made other modifications. Yet the result so far is underwhelming: even after genome-wide edits (in one case over 60 edits, and in another using over 13,000 simultaneous base edits!), the pig organs still fail to thrive in primate tests, and the longest-lasting pig-to-primate organ transplants came from pigs with far fewer edits edepot.wur.nledepot.wur.nl. The science is still catching up to the biological complexity – removing one set of immunogenic genes often reveals other factors that cause rejection or dysfunction. As commentators noted, this “techno-capacity” for extensive editing has not overcome the fundamental complex interactions of biology, which defy our ability to predict outcomes edepot.wur.nl. Similarly, plant biologists have found that ostensibly precise CRISPR edits in crops can produce unexpected offshoot mutations if you look closely. One 2020 FDA analysis of gene-edited plants and animals uncovered a slew of insertions, deletions, and rearrangements that standard screening methods had missed edepot.wur.nledepot.wur.nl. Scientists had to develop new genome sequencing techniques to even detect these errors, underscoring that conventional checks were “falsely claiming” many edited genomes were clean news-medical.net. In essence, our toolbox for understanding and verifying gene function is lagging behind our ability to make changes. We can cut DNA efficiently, but we still struggle to anticipate or even detect all the consequences, thanks to the sheer complexity of the genome’s regulation and our incomplete knowledge.
3. Overhyped Cases and Unmet Promises
Gene editing has been heralded as a revolution – with talk of curing genetic diseases, creating super-crops, and even bringing back extinct species. But a number of high-profile cases show a pattern of hype outpacing reality:
Gene-Edited Hornless Cattle: This was touted as a poster child for agricultural gene editing – a precise edit to introduce a harmless trait (cows without horns, to improve animal welfare) without any transgenic DNA. The result was initially celebrated as a success. However, when FDA scientists rigorously examined the calves’ genome, they found an unintended DNA surprise. The editing process had inadvertently inserted bacterial plasmid sequences into the cow’s genome, including two genes for antibiotic resistance edepot.wur.nl. In other words, the “precisely edited” cow carried foreign DNA that would never occur naturally, contradicting the developers’ claim that the animal was the same as a conventionally bred cow. This discovery – made by chance during testing of a new analysis software – cast serious doubt on the “slam-dunk” precision claims of such edits thecounter.org. It led to calls for a pause and more oversight, and the breeding program for these cattle was halted once the error came to light news-medical.netnews-medical.net. The hornless cattle case illustrates how initial hype (“look, we edited one gene and solved a problem”) gave way to recognition of hidden pitfalls. Things “can go wrong that you don’t intend to happen, and they’re not always detected,” as an FDA official said in the aftermath thecounter.orgthecounter.org.
CRISPR Baby Scandal: The creation of CRISPR-edited babies in China was trumpeted by its creator as a historic leap – the babies were supposed to be genetically protected against HIV. Instead, it became a global scandal and a scientific cautionary tale. Beyond the ethical firestorm, the scientific outcome was far from the “precise medical benefit” that was advertised. The twins ended up with unknown mutations in the target gene (CCR5) – not the well-understood HIV-resistant variant, but novel disruptions with unpredictable effects journals.plos.org. In fact, only some of their cells had the intended edits (one twin was mosaic, carrying a mix of edited and unedited cells) journals.plos.org. Prominent researchers lambasted the effort as reckless and pointless, noting there were much safer ways to prevent HIV in those families theatlantic.comtheatlantic.com. The “CRISPR babies” case demonstrates how the promised outcome did not materialise: instead of healthy, HIV-proof children, we have children whose genomes were altered in unprecedented ways with no clear benefit – and a legacy of eroded public trust. It’s a prime example of CRISPR’s precision being overstated: the editing was supposed to hit one gene and improve it, but missed the mark both scientifically and morally.
Medical Treatments and Trials: In medicine, the narrative around CRISPR has been incredibly optimistic – from magazine covers to Nobel Prize press releases declaring it “may make the dream of curing inherited diseases come true.” statnews.com
Billions of dollars have flowed into CRISPR biotech startups, and patients have been led to expect miracles. Yet so far, the tangible outcomes are limited. The first CRISPR-based therapies (for sickle cell disease and a few other blood disorders) are just now approaching approva, but they involve ex vivo cell editing (editing cells outside the body) and come with steep costs and risks. Many other early CRISPR human trials have struggled or been delayed. For example, Editas Medicine’s much-hyped trial to cure a form of blindness by directly editing DNA in the eye reported only modest, ambiguous results in a few patients. Other companies found that delivering CRISPR into the body and getting it to the right cells is far harder than the hype implied. Even the “success stories” carry caveats – CRISPR Therapeutics had a “breakthrough” in sickle cell, but shortly before its therapy achieved approval, the company unexpectedly laid off staff, an early sign that “for all the public accolades, the CRISPR revolution wasn’t exactly going according to plan.” statnews.com
Industry observers note that the gene editing field is entering a more sober phase as it grapples with practical challenges and safety issues. As STAT News reported in 2025, “The CRISPR companies are not OK” – hype and scientific setbacks have humbled the industry and cut into the once sky-high investor confidence statnews.com.
In short, we have yet to see the CRISPR equivalent of a penicillin. Instead, we see incremental advances, high costs, and a growing realisation that biological complexities make easy cures unlikely.“Precise” Trait Editing in Crops and Animals: Beyond the hornless cows, many gene-edited agriculture projects have seen mixed outcomes. Tomato plants edited to yield more or grow in harsher climates sometimes showed stunted growth or other unintended trait changes, requiring researchers to backtrack. Pigs edited to be resistant to a viral disease ended up with compromised immune function in other areas. In one well-publicized case, researchers created “double-muscle” pigs by deleting the myostatin gene (similar to the beagle experiment above), and while the pigs were indeed muscular, some had enlarged tongues or other deformities, and breeding those pigs proved difficult. These stories rarely make headlines in the way the initial “CRISPR success” does. They highlight a common pattern: over-promising and under-delivering.
4. Critiques of Overmarketing and the Biomarker Analogy
Amid the excitement, a number of experts and watchdogs have urged caution, warning that gene editing is following the same hype cycle that earlier biotech trends did – notably the “biomarker boom” of the 2000s. In that era, researchers and companies raced to identify blood or genetic biomarkers for every disease (to enable early diagnosis and targeted treatment), and investors poured money in. While that field yielded some useful tests, it largely overpromised – many purported biomarkers turned out to be unreliable or not clinically useful, and the flood of publications failed to translate into commensurate health outcomes. As a 2004 Journal of the NCI commentary put it, the biomarker boom was “slowed by validation concerns”, when many early claims couldn’t be validated in larger studies pubmed.ncbi.nlm.nih.gov. In other words, the science had to catch up with the hype, and much of the initial optimism had to be recalibrated.
A similar dynamic is at play with gene editing. Thoughtful commentators note that the public narrative around CRISPR has been deliberately narrow and glossy, focusing on best-case scenarios and “simple” fixes. A 2021 academic analysis argued that CRISPR’s image is built on a “strategically narrow narrative that omits the complexity and uncertainty of postgenomic research” edepot.wur.nl. By presenting gene editing as precise, predictable, and nearly infallible, proponents have generated public support and investor enthusiasm – but at the cost of creating unrealistic expectations edepot.wur.nledepot.wur.nl. This narrative casts the gene as a stable, controllable object (pressuring regulators to treat edited organisms lightly), whereas actual genomics shows genes operate in a “higgledy-piggledy” network of feedback loops and environmental interactions edepot.wur.nledepot.wur.nl. As the Elementa article pointed out, emphasizing the “ease and precision” of CRISPR while glossing over the “complexity of the gene” is a form of marketing that can backfire edepot.wur.nledepot.wur.nl. It feeds the hype cycle in the short term but undermines public trust when the oversimplifications are later exposed by real-world results edepot.wur.nledepot.wur.nl.
Commercialisation Pressure: The rapid commercialization of CRISPR has also drawn critique. By 2025, multiple investigative pieces noted that gene editing startups were struggling to meet the lofty goals set in their splashy IPOs and media campaigns. STAT News described how “hype, scientific setbacks, and growing investor demands humbled the gene editing industry”statnews.com. Companies found that the timelines for cures were longer and more uncertain than investors were led to believe. This mirrors the trajectory of the genomic biomarker companies a decade earlier – a surge of investment followed by a sobering phase of technical hurdles and financial corrections. Some analysts have started warning that CRISPR tech is entering the “trough of disillusionment” in the classic Gartner hype cycle, where inflated expectations collide with the arduous process of product development and regulation.
In sum, credible voices in science and medicine are urging a recalibration of expectations. Gene editing is a powerful tool, but it is not magic. As one research director quipped, “New myths about the powers of CRISPR are bound to keep popping up… and they usually have a grain of truth in them.” idtdna.com
The grain of truth is that CRISPR can indeed make targeted changes to DNA; the myth is that we can predict and control the outcomes with blueprint-like accuracy. Drawing parallels to the biomarker boom (and subsequent bust) provides a valuable sense of humility: it reminds us that biology is extraordinarily complex, and that early excitement must be tempered with rigorous validation. Otherwise, we risk repeating history – overselling a technology, underestimating the challenges, and then facing public backlash when the “miracles” take longer to materialise.
Each innovation’s true impact often becomes clear only after the hype dust settles – and for CRISPR, that dust is only just beginning to settle now.
SEE sources and citations below.
🚨 Magic Pills // The microbiome is a reflection of your health — not a cure!
You can’t poison your body and expect microbes to save you. They mirror your internal environment; they don’t override it.
Sources:
Shah et al., Elementa (2021) – Analysis of the “narrow narrative” of CRISPR vs. gene complexityedepot.wur.nledepot.wur.nledepot.wur.nledepot.wur.nl.
Antoniou, in Gene Editing Myths report (2020) – Expert critique that gene editing isn’t proven more precise than traditional breedinggreens-efa.eugreens-efa.eu.
Science News (2015) – Report on CRISPR-edited beagle dogs, noting low efficiency and mirroring of a natural mutationsciencenews.orgsciencenews.org.
The Counter (2019) – Investigation of gene-edited hornless cattle, revealing unintended antibiotic-resistance genes and calling “slam-dunk” claims into questionthecounter.orgthecounter.org.
News-Medical (2020) – Summary of studies showing gene editing’s error-prone outcomes and detection challengesnews-medical.netnews-medical.netnews-medical.net.
PLOS Biology (2019) – Commentary on the CRISPR babies experiment, detailing the lack of medical necessity and the off-target/unintended effects in CCR5-edited childrenjournals.plos.orgjournals.plos.org.
STAT News (2025) – “The CRISPR companies are not OK” report on how hype and setbacks humbled the industrystatnews.comstatnews.comstatnews.com.
FDA CVM study via Nature Biotech (2020) – Findings of large unintended mutations in edited genomes (e.g., cattle)edepot.wur.nledepot.wur.nl.
JNCI (2004) – “Biomarker boom slowed by validation concerns,” highlighting the previous cycle of hype in biotechpubmed.ncbi.nlm.nih.gov.
Others as cited in-line above (Science/Atlantic reports on CRISPR, etc.) for additional context and commentarytheatlantic.comthecounter.org.
Citations:
FDA finds surprise in gene-edited cattle: Antibiotic-resistant, non-cow DNA
https://thecounter.org/fda-gene-edited-cattle-antibiotic-resistant-crispr-dna/
FDA finds surprise in gene-edited cattle: Antibiotic-resistant, non-cow DNA
https://thecounter.org/fda-gene-edited-cattle-antibiotic-resistant-crispr-dna/
FDA finds surprise in gene-edited cattle: Antibiotic-resistant, non-cow DNA
https://thecounter.org/fda-gene-edited-cattle-antibiotic-resistant-crispr-dna/
Gene-editing is more error-prone than thought, new findings suggest
https://www.greens-efa.eu/files/assets/docs/geneeditingmyths_report_a4_v4_web_reduced.pdf
https://www.greens-efa.eu/files/assets/docs/geneeditingmyths_report_a4_v4_web_reduced.pdf
Muscle-gene edit creates buff beagles
https://www.sciencenews.org/article/muscle-gene-edit-creates-buff-beagles
Muscle-gene edit creates buff beagles
https://www.sciencenews.org/article/muscle-gene-edit-creates-buff-beagles
Muscle-gene edit creates buff beagles
https://www.sciencenews.org/article/muscle-gene-edit-creates-buff-beagles
Gene-edited babies: What went wrong and what could go wrong | PLOS Biology
https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000224
Gene-edited babies: What went wrong and what could go wrong | PLOS Biology
https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000224
Gene-editing is more error-prone than thought, new findings suggest
Gene-editing is more error-prone than thought, new findings suggest
Gene-editing is more error-prone than thought, new findings suggest
FDA finds surprise in gene-edited cattle: Antibiotic-resistant, non-cow DNA
https://thecounter.org/fda-gene-edited-cattle-antibiotic-resistant-crispr-dna/
FDA finds surprise in gene-edited cattle: Antibiotic-resistant, non-cow DNA
https://thecounter.org/fda-gene-edited-cattle-antibiotic-resistant-crispr-dna/
First Gene-Edited Babies Have Allegedly Been Born in China - The Atlantic
First Gene-Edited Babies Have Allegedly Been Born in China - The Atlantic
The CRISPR gene editing revolution loses its mojo | STAT
The CRISPR gene editing revolution loses its mojo | STAT
The CRISPR gene editing revolution loses its mojo | STAT
Biomarker boom slowed by validation concerns - PubMed
https://pubmed.ncbi.nlm.nih.gov/15367567/
The hype behind gene editing: Myth busting CRISPR
https://www.idtdna.com/pages/community/blog/post/the-hype-behind-gene-editing-myth-busting-crispr
Gene-editing is more error-prone than thought, new findings suggest
Gene-edited babies: What went wrong and what could go wrong | PLOS Biology
https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000224